Atomic Mass and Nuclear Binding Energy for S-33 (Sulfur)
Abstract
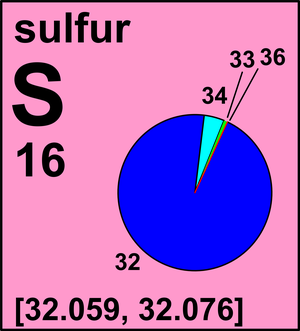
Atomic Number: 16 Atomic Mass: 32.066 amu Melting Point: 112.8 °C (385.95 K, 235.04001 °F) Boiling Point: 444.6 °C (717.75 K, 832.28 °F) Number of Protons/Electrons: 16 Number of Neutrons: 16 Classification: Non-metal Crystal Structure: Orthorhombic Density @ 293 K: 2.07 g/cm 3 Color: yellow British Spelling: Sulphur IUPAC Spelling: Sulfur. Sulfur (in traditional lay Commonwealth English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8. Atomic Data for Sulfur (S) Atomic Number = 16 Atomic Weight = 32.066 Reference E95: Isotope: Mass: Abundance: Spin: Mag Moment: 32 S: 31.972070: 95.02%.
Sulfur Atomic Mass And Number
This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope S-33 (Sulfur, atomic number Z = 16, mass number A = 33).
Sulfur Atomic Mass And Atomic Number
- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
